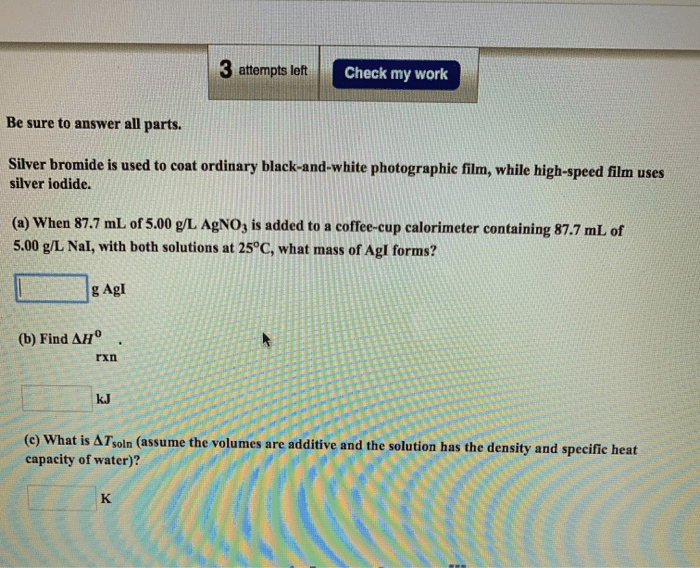
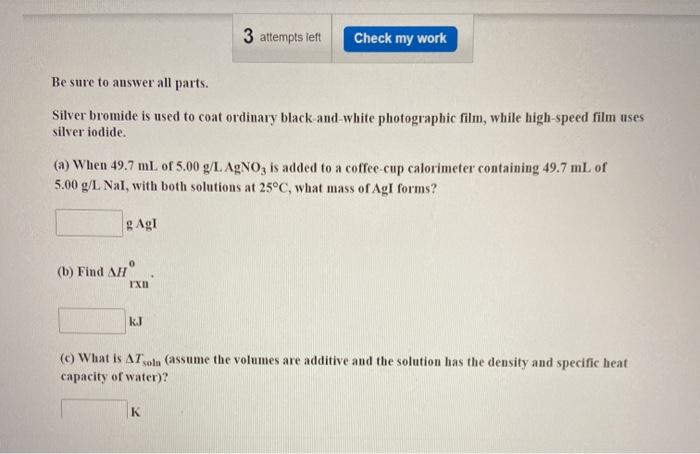
Silver bromide is used to coat ordinary black-and-white – Silver bromide, a cornerstone of black-and-white photography, has played a pivotal role in capturing and preserving countless moments in history. Its unique properties and versatile applications have made it an enduring choice for photographers seeking to create timeless images.
Silver bromide is a light-sensitive compound that forms the basis of photographic emulsions. When exposed to light, silver bromide undergoes a chemical reaction that creates a latent image. This latent image can then be developed to produce a visible, permanent image.
History of Silver Bromide in Photography

Silver bromide, a light-sensitive compound, has played a pivotal role in the development of photography. Its discovery in the mid-19th century marked a significant advancement, leading to the creation of black-and-white film and paper.
In 1840, John Herschel first described the light-sensitive properties of silver bromide. However, it was not until the work of Richard Leach Maddox in 1871 that silver bromide was successfully used to coat photographic plates. This innovation allowed for longer exposure times, making photography more practical.
The development of roll film in the late 19th century further revolutionized photography, making it more accessible to the general public. Silver bromide was the primary light-sensitive material used in these films, and it remained the dominant choice for black-and-white photography until the advent of digital cameras.
Properties of Silver Bromide
Silver bromide is a crystalline compound with the chemical formula AgBr. It is a highly light-sensitive material, meaning that when exposed to light, it undergoes a chemical reaction that produces silver atoms. These silver atoms form the basis of the latent image in photography.
The sensitivity of silver bromide to light can be controlled by the size and shape of its crystals. Smaller crystals are more sensitive to light than larger crystals. The size and shape of the crystals can be controlled during the manufacturing process, allowing for the production of emulsions with varying sensitivities.
Manufacturing Process of Silver Bromide Emulsions
Silver bromide emulsions are produced by a complex manufacturing process that involves several steps:
- Silver nitrate and potassium bromide are dissolved in water to form a solution.
- The solution is heated to a controlled temperature, and a gelatin solution is added.
- The gelatin solution acts as a binder, holding the silver bromide crystals in suspension.
- The emulsion is then cooled and coated onto a support material, such as film or paper.
The manufacturing process can be controlled to produce emulsions with different sensitivities and grain sizes. Emulsions with smaller grain sizes produce finer-grained images, while emulsions with larger grain sizes produce more coarse-grained images.
Use of Silver Bromide in Black-and-White Photography
In black-and-white photography, silver bromide is used to form the latent image. When light strikes the emulsion, it causes the silver bromide crystals to undergo a chemical reaction that produces silver atoms. These silver atoms form the basis of the latent image.
The latent image is invisible, but it can be made visible by a process called development. During development, the latent image is exposed to a chemical solution that converts the silver atoms into black silver. The resulting image is a negative, in which the dark areas of the image correspond to the light areas of the scene, and vice versa.
Comparison of Silver Bromide to Other Photographic Materials
Silver bromide is one of several light-sensitive materials that have been used in photography. Other materials include silver chloride, silver iodide, and gelatin silver.
Silver bromide is more sensitive to light than silver chloride, making it more suitable for use in low-light conditions. However, silver chloride produces a finer-grained image than silver bromide.
Silver iodide is less sensitive to light than silver bromide, but it is more sensitive to blue light. This makes it useful for creating images with a wide tonal range.
Environmental and Health Considerations, Silver bromide is used to coat ordinary black-and-white
Silver bromide is a hazardous material, and it can pose environmental and health risks if not handled properly. Silver bromide waste can contaminate water sources, and it can also be harmful to aquatic life.
Proper disposal methods for silver bromide waste include recycling, landfilling, and incineration. Recycling is the preferred method, as it allows the silver to be recovered and reused.
Question Bank: Silver Bromide Is Used To Coat Ordinary Black-and-white
What is the role of silver bromide in black-and-white photography?
Silver bromide forms the basis of photographic emulsions, which are light-sensitive coatings applied to photographic film and paper. When exposed to light, silver bromide undergoes a chemical reaction that creates a latent image, which can then be developed to produce a visible, permanent image.
How is silver bromide manufactured?
Silver bromide is manufactured through a complex process involving the reaction of silver nitrate and potassium bromide in a gelatin solution. The resulting emulsion is then coated onto a film or paper base.
What are the advantages of using silver bromide in photography?
Silver bromide offers several advantages for photography, including its high sensitivity to light, its ability to capture fine detail, and its wide tonal range. It is also a relatively stable compound, making it suitable for long-term storage.