Pogil properties of covalent bonds – The realm of covalent bonding, where atoms share electrons to form stable molecules, presents a fascinating array of properties that govern the behavior of matter. This exploration delves into the polarity, bond length, bond order, hybridization, molecular geometry, and resonance of covalent bonds, unraveling their intricate interplay and profound influence on molecular structure and reactivity.
From the polarity that arises from electronegativity differences to the bond strength determined by bond order and resonance, the properties of covalent bonds provide a comprehensive understanding of the forces that shape the molecular world.
FAQ Insights: Pogil Properties Of Covalent Bonds
What is the significance of electronegativity in covalent bonding?
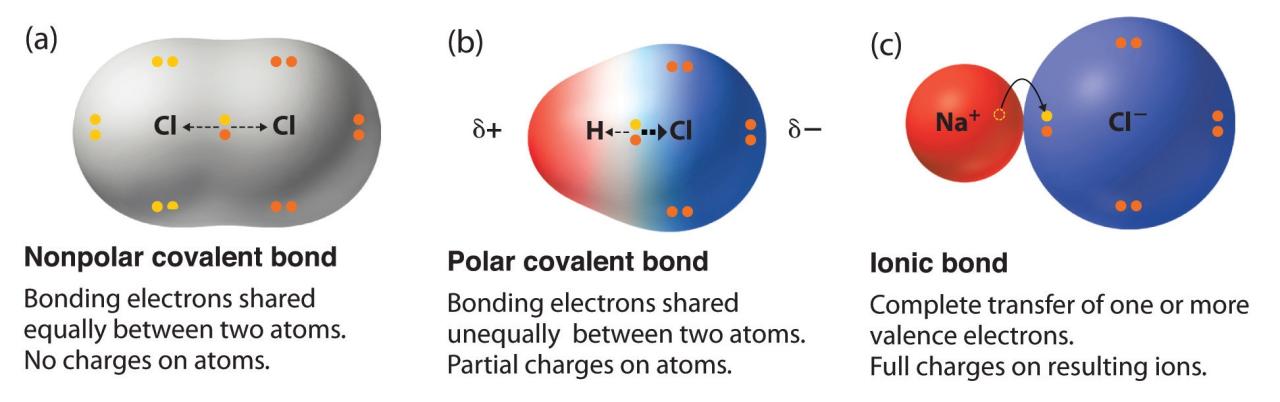
Electronegativity determines the polarity of covalent bonds, influencing the distribution of electrons and the formation of partial charges.
How does bond order affect bond strength?
Bond order is directly proportional to bond strength; higher bond orders indicate stronger bonds.
What is the role of hybridization in determining molecular geometry?
Hybridization involves the mixing of atomic orbitals to form hybrid orbitals, which dictate the arrangement of atoms in a molecule, determining its geometry.
How does resonance contribute to molecular stability?
Resonance involves the delocalization of electrons across multiple bonds, resulting in resonance structures that contribute to the overall stability of the molecule.