Embark on a comprehensive exploration of Le Chatelier’s Principle Virtual Lab Answers, a topic that unveils the intricacies of chemical reactions and their response to external influences. This principle serves as a fundamental concept in chemistry, providing a framework to understand and predict the behavior of chemical systems under varying conditions.
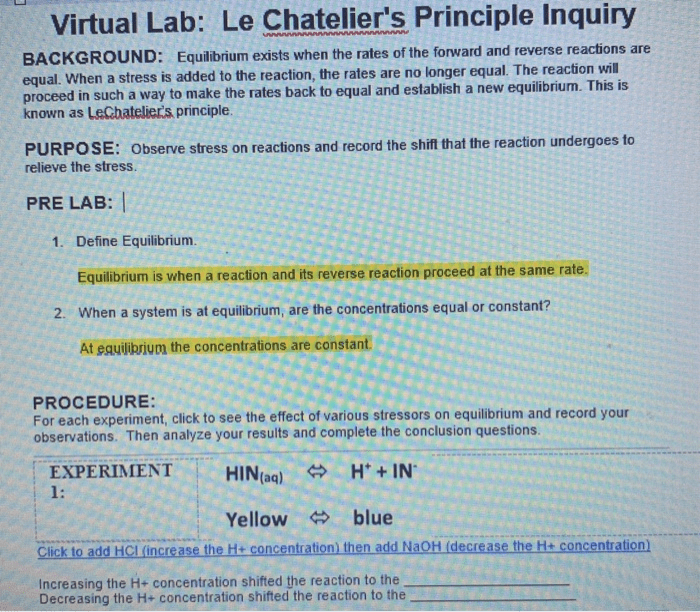
Virtual labs offer an innovative approach to studying Le Chatelier’s principle. They provide a safe and interactive environment to simulate chemical reactions and observe the effects of changing parameters, allowing students and researchers to gain a deeper understanding of the concept.
Le Chatelier’s Principle: Le Chatelier’s Principle Virtual Lab Answers

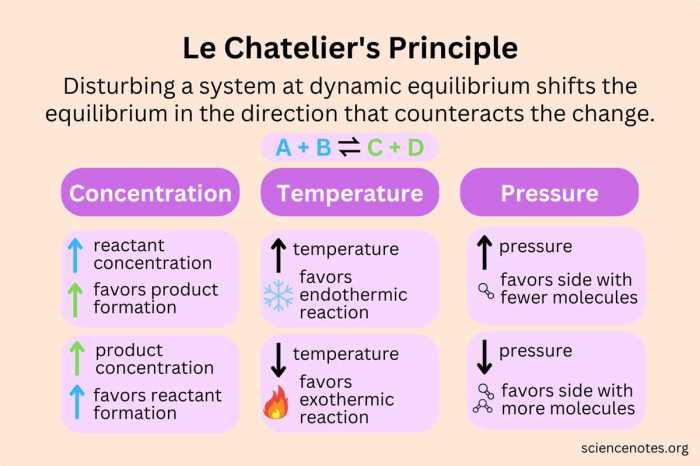
Le Chatelier’s principle is a fundamental concept in chemistry that describes the behavior of chemical systems when they are subjected to changes in temperature, pressure, or concentration. It states that if a change is applied to a system at equilibrium, the system will shift in a direction that counteracts the change.
For example, if the concentration of a reactant is increased, the system will shift to the product side to reduce the concentration of the reactant. Similarly, if the temperature is increased, the system will shift to the side of the reaction that absorbs heat.
Virtual Lab Experiments
Virtual labs offer a safe and convenient way to study Le Chatelier’s principle. They allow students to simulate chemical reactions and observe the effects of changes in temperature, pressure, and concentration on the equilibrium position.
Virtual labs can be used to:
- Visualize the changes that occur in a chemical reaction at equilibrium
- Test the predictions of Le Chatelier’s principle
- Explore the effects of different variables on the equilibrium position
Analyzing Results, Le chatelier’s principle virtual lab answers
The results of virtual lab experiments can be analyzed using graphs and tables to identify the effects of Le Chatelier’s principle.
Graphs can be used to show how the concentration of reactants and products changes over time. Tables can be used to summarize the data and show the equilibrium concentrations of the reactants and products.
Applications of Le Chatelier’s Principle
Le Chatelier’s principle has a wide range of applications in chemistry, industry, and medicine.
In chemistry, Le Chatelier’s principle is used to:
- Optimize chemical reactions
- Predict the products of a reaction
- Control the selectivity of a reaction
In industry, Le Chatelier’s principle is used to:
- Maximize the yield of a reaction
- Control the quality of a product
- Design new processes
In medicine, Le Chatelier’s principle is used to:
- Develop new drugs
- Optimize drug delivery
- Understand the effects of drugs on the body
Limitations of Le Chatelier’s Principle
Le Chatelier’s principle is a general principle that can be applied to a wide range of chemical reactions. However, there are some limitations to its applicability.
Le Chatelier’s principle does not apply to:
- Reactions that are not at equilibrium
- Reactions that are very slow
- Reactions that involve multiple equilibria
FAQ Guide
What is Le Chatelier’s principle?
Le Chatelier’s principle states that if a change of condition is applied to a system in equilibrium, the system will shift in a direction that relieves the stress.
How can virtual labs be used to study Le Chatelier’s principle?
Virtual labs allow students to simulate chemical reactions and observe the effects of changing parameters, such as temperature, pressure, and concentration, on the equilibrium position.
What are the limitations of Le Chatelier’s principle?
Le Chatelier’s principle does not apply to all chemical reactions and may not accurately predict the direction of the reaction in certain cases, such as reactions involving weak acids or bases or reactions with a large number of reactants and products.