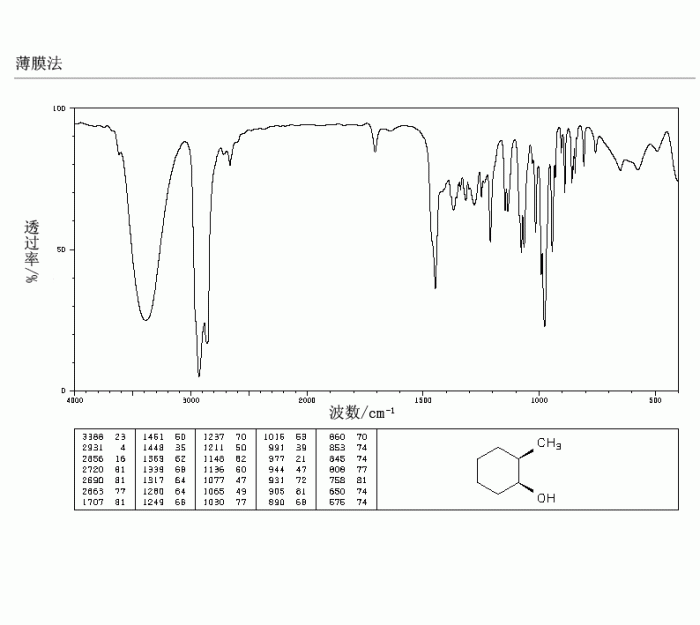
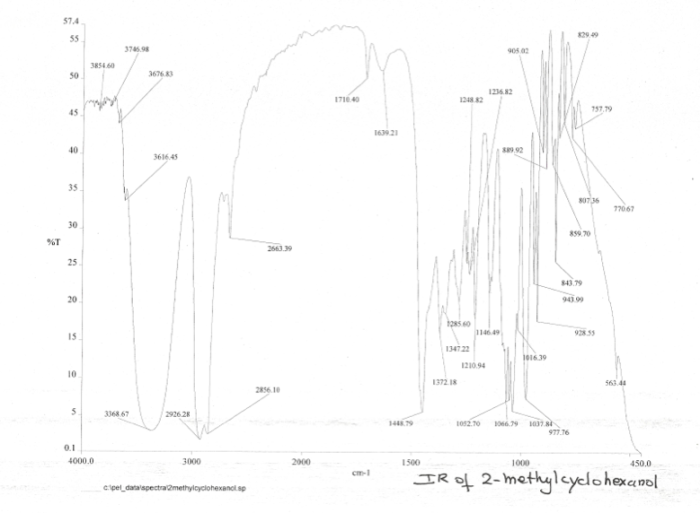
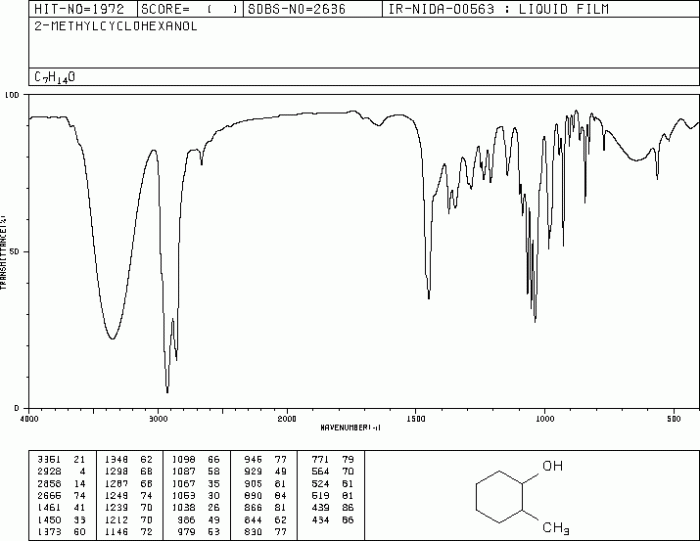
Ir spectrum of 2 methylcyclohexanol – The IR spectrum of 2-methylcyclohexanol, a valuable tool for organic compound analysis, unveils the intricate molecular structure and functional group composition of this versatile alcohol. Delving into the principles of IR spectroscopy, we embark on a journey to decipher the characteristic absorption bands that paint a vivid picture of 2-methylcyclohexanol’s molecular identity.
The IR spectrum of 2-methylcyclohexanol serves as a fingerprint, providing a wealth of information about its structural features. Key functional groups, such as the hydroxyl group and the cyclohexane ring, leave their unique spectral signatures, allowing us to identify and characterize this compound with precision.
IR Spectrum of 2-Methylcyclohexanol
Infrared (IR) spectroscopy is a powerful analytical technique used to identify and characterize organic compounds. It involves the absorption of infrared radiation by functional groups within the molecule, resulting in specific vibrational modes that can be detected and analyzed.
Key Functional Groups in 2-Methylcyclohexanol
2-Methylcyclohexanol contains the following key functional groups:
- Alcohol (OH) group
- Cyclohexane ring (C-H bonds)
- Methyl group (CH 3)
Characteristic IR Absorption Bands
The IR spectrum of 2-methylcyclohexanol exhibits the following characteristic absorption bands:
- 3300-3650 cm-1: O-H stretching vibration (alcohol group)
- 2850-3000 cm-1: C-H stretching vibrations (cyclohexane ring and methyl group)
- 1000-1300 cm-1: C-O stretching vibration (alcohol group)
Structural Analysis
The IR spectrum confirms the presence of the alcohol group, cyclohexane ring, and methyl group in 2-methylcyclohexanol. The absorption bands at 3300-3650 cm -1and 1000-1300 cm -1indicate the presence of the alcohol group, while the absorption bands at 2850-3000 cm -1indicate the presence of the cyclohexane ring and methyl group.
Comparison with Other Alcohols, Ir spectrum of 2 methylcyclohexanol
The IR spectrum of 2-methylcyclohexanol differs from that of other alcohols due to the presence of the cyclohexane ring. The cyclohexane ring introduces additional C-H stretching vibrations in the 2850-3000 cm -1region, which are not present in other alcohols.
Applications
IR spectroscopy is widely used in the analysis of 2-methylcyclohexanol for various applications, including:
- Identification and characterization of 2-methylcyclohexanol in quality control and research settings
- Forensic analysis to identify unknown substances
- Monitoring of chemical reactions involving 2-methylcyclohexanol
Expert Answers: Ir Spectrum Of 2 Methylcyclohexanol
What is the most prominent IR absorption band in the spectrum of 2-methylcyclohexanol?
The O-H stretching vibration typically gives rise to a broad and intense absorption band in the region of 3300-3600 cm-1.
How can IR spectroscopy differentiate between primary, secondary, and tertiary alcohols?
The position and intensity of the O-H stretching band provide valuable information. Primary alcohols exhibit a sharp band near 3650 cm-1, secondary alcohols show a broader band around 3500 cm-1, and tertiary alcohols lack a distinct O-H stretching band.